What Is Kidney Cancer?
Kidney cancer is a type of cancer that starts in the kidney when cells in the body begin to grow out of control. As more cancer cells develop, they can form a tumor and, with time, might spread to other body parts.
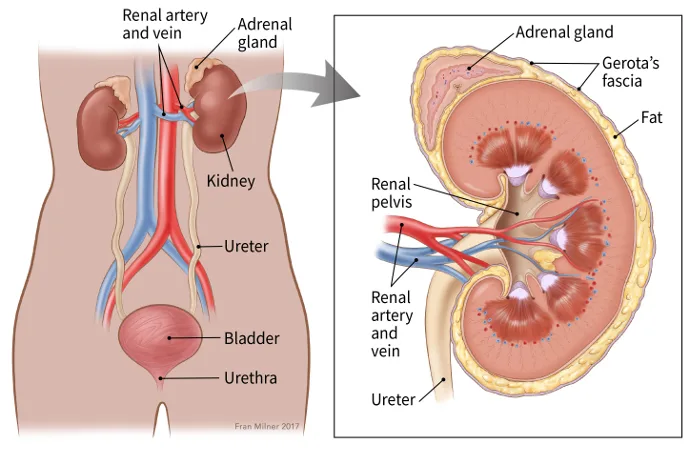
The kidneys
The kidneys are a pair of bean-shaped organs, each about the size of a fist. They are attached to the upper back wall of the abdomen and are protected by the lower rib cage. One kidney is just to the left and the other just to the right of the backbone.
An adrenal gland sits on top of each kidney. Each kidney and adrenal gland is surrounded by fat and a thin, fibrous layer known as Gerota’s fascia.
The kidneys’ main job is to remove excess water, salt, and waste products from blood coming in from the renal arteries. These substances become urine. Urine collects in the center of each kidney in an area called the renal pelvis and then leaves the kidneys through long, slender tubes called ureters. The ureters lead to the bladder, where the urine is stored until you urinate.
The kidneys also have other jobs:
- They make a hormone called renin that helps control blood pressure.
- They help make sure the body has enough red blood cells by making a hormone called erythropoietin. This hormone tells the bone marrow to make more red blood cells.
Our kidneys are important, but our bodies can function with only one kidney. Many people in the United States are living normal, healthy lives with just one kidney.
Some people do not have working kidneys at all and live with the help of a medical procedure called dialysis. The most common form of dialysis uses a specially designed machine that filters blood much like a real kidney would.
Types of kidney cancer
Not all cancers that start in the kidneys are the same. Different types of kidney cancer can act differently and might need different treatments.
Renal cell carcinoma
Renal cell carcinoma (RCC), also known as renal cell cancer, is the most common type of kidney cancer. About 9 out of 10 kidney cancers are renal cell carcinomas. If you’re told you have kidney cancer, it’s most likely to be a renal cell carcinoma.
Although RCC usually grows as a single tumor within a kidney, sometimes a person can have more than one tumor in a kidney or even tumors in both kidneys at the same time.
There are several subtypes of RCC. Knowing the subtype of RCC can help decide on treatment and can also help your doctor figure out if your cancer might be caused by an inherited genetic syndrome.
Clear cell renal cell carcinoma
This is the most common form of RCC. About 7 out of 10 people with RCC have this kind of cancer. When seen with a microscope, the cells that make up clear cell RCC look very pale or clear.
Non-clear cell renal cell carcinomas
These include all subtypes that are not clear cell RCCs.
Papillary renal cell carcinoma: About 1 in 10 RCCs are of this type. These cancers form little finger-like projections (called papillae) in some, if not most, of the tumors. Some doctors call these cancers chromophilic because the cells take in certain dyes and look pink when seen with a microscope.
Chromophobe renal cell carcinoma: This subtype accounts for about 5% (5 cases in 100) of RCCs. The cells of these cancers are pale, like the clear cells, but are darker and have certain other features that can be recognized when looked at very closely.
Rare subtypes of renal cell carcinoma: Several other subtypes each make up less than 1% of RCCs. These include:
- Collecting duct RCC
- Multilocular cystic RCC
- Medullary carcinoma (renal medullary carcinoma)
- Mucinous tubular and spindle cell carcinoma
- Neuroblastoma-associated RCC
There are some other rare subtypes as well, which have certain gene or chromosome changes inside the cancer cells.
Unclassified renal cell carcinoma: Some RCCs are labeled as ‘unclassified’ because the way they look doesn’t fit into any of the other categories or because there is more than one type of cancer cell present. These account for about 5% of RCCs.
Other types of kidney cancers
Other types of kidney cancers include transitional cell carcinomas, Wilms tumors, and renal sarcomas.
Transitional cell carcinoma
Of every 100 cancers in the kidney, about 5 to 10 are transitional cell carcinomas (TCCs), also known as urothelial carcinomas.
Transitional cell carcinomas start in the lining of the renal pelvis (where the ureters meet the kidneys). This lining is made up of cells called transitional cells, which are the same cells that line the ureters and bladder. Cancers that develop from these cells look like other urothelial carcinomas, such as bladder cancer, when looked at closely. Like bladder cancer, these cancers are often linked to cigarette smoking and being exposed to certain cancer-causing chemicals in the workplace.
People with TCC often have the same signs and symptoms as people with renal cell cancer − blood in the urine and, sometimes, back pain.
Wilms tumor (nephroblastoma)
Wilms tumors almost always occur in children. This type of cancer is very rare in adults.
Renal sarcoma
Renal sarcomas are a rare type of kidney cancer that begins in the blood vessels or connective tissue of the kidney. They make up less than 1% of all kidney cancers.
Benign (non-cancerous) kidney tumors
Some kidney tumors are benign (not cancerous). They don’t metastasize (spread) to other parts of the body, although they can still grow and cause problems.
Benign kidney tumors can usually be treated, if needed, by removing or destroying them. This can be done with many of the same treatments that are also used for kidney cancers, such as surgery or radiofrequency ablation (RFA). The choice of treatment depends on many factors, such as the size of the tumor and if it is causing symptoms, the number of tumors, if there are tumors in both kidneys, and a person’s general health.
Angiomyolipoma
Angiomyolipomas are the most common type of benign kidney tumor. They are seen more often in women. They can develop sporadically or in people with tuberous sclerosis, a genetic condition that also affects the heart, eyes, brain, lungs, and skin.
These tumors are made up of different types of connective tissues (blood vessels, smooth muscles, and fat). If they aren’t causing any symptoms, they can often just be watched closely. If they start causing problems (like pain or bleeding), they may need to be treated.
Oncocytoma
Oncocytomas are uncommon benign kidney tumors that can sometimes grow quite large. They are seen more often in men and do not normally spread to other organs. Surgery usually cures them. The rest of the information here is written about kidney cancer, focusing on renal cell carcinoma and not on less common types of kidney tumors.
Key Statistics About Kidney Cancer
Kidney cancer is one of the 10 most common cancers in both men and women in the United States. It accounts for about 4% to 5% of all cancers.
How many people get kidney cancer?
The American Cancer Society’s most recent estimates for kidney cancer in the United States for 2024 are:
- About 81,610 new cases of kidney cancer (52,380 in men and 29,230 in women) will be diagnosed.
- About 14,390 people (9,450 men and 4,940 women) will die from this disease
These numbers include all types of kidney and renal pelvis cancers.
Most people with kidney cancer are older. The average age of people when they are diagnosed is 65, with most people being diagnosed between the ages of 55 and 74. Kidney cancer is uncommon in people younger than age 45.
Kidney cancer is about twice as common in men as in women, and it is more common in African American, American Indian, and Alaska Native people.
Lifetime risk of kidney cancer
Overall, the lifetime risk for developing kidney cancer in men is about 1 in 43 (2.3%). The lifetime risk for women is about 1 in 73 (1.4%). But each person’s risk can be affected by a number of factors.
Trends in new cases and death rates
The rate of new kidney cancers found each year has been rising for many years. Part of this rise has probably been due to the use of newer imaging tests such as CT scans, which have picked up some cancers that might never have been found otherwise.
On the other hand, death rates for kidney cancer have been falling for many years.
What’s New in Kidney Cancer Research?
Research into the causes, detection, diagnosis, and treatment of kidney cancer (renal cell carcinoma, or RCC) is being done at many medical centers, university hospitals, and other institutions around the world. A few examples of these are discussed here.
Genetics of kidney cancer
Researchers are learning more about the gene changes inside kidney cells that cause them to become cancer cells. Knowing about these changes has helped doctors better classify kidney cancers into different types, which can sometimes affect treatment choices. This information has also helped researchers develop newer drugs that specifically target some of these changes inside the cancer cells.
Local forms of treatment
Many kidney cancers can be treated by removing or destroying the tumor(s). Treatments such as surgery, ablative treatments, and radiation therapy can be helpful in different situations. Doctors are developing newer approaches to these treatments. For example:
Surgery to remove kidney cancer can now often be done using robotic-assisted laparoscopic surgery, in which the surgeon sits at a console in the operating room and controls very precise robotic arms to do the surgery.
Newer types of ablative treatments, such as microwave ablation and irreversible electroporation, are now being studied for use in destroying tumors in the kidneys or other parts of the body.
Newer forms of radiation therapy, such as stereotactic body radiation therapy (SBRT), are now an option to treat some tumors.
Treatment options for advanced non-clear cell renal cell carcinoma
The most common type of kidney cancer is clear cell RCC. A small portion of kidney cancers, called non-clear cell RCCs, are different subtypes, which might respond better to different types of treatment than those used for clear cell RCC. Doctors are now studying which treatments might be most helpful for these cancers.
Targeted therapy and immunotherapy
In the last couple of decades, newer types of drug treatments have emerged as the most effective treatment options for the most advanced kidney cancers.
Targeted drugs attack specific parts of cancer cells (or nearby cells that help them grow, such as the cells that make new tumor blood vessels).
Immunotherapy drugs help the body’s immune system find and attack the cancer cells.
These types of drugs, alone or combined, are now usually the main treatment options for kidney cancers that can’t be removed completely with surgery. Doctors are now studying which combinations of drugs might work best.
Newer types of targeted and immunotherapy drugs are being developed. For example, CAR T-cells are a person’s immune cells that have been altered in a lab to attack a target on cancer cells. Researchers are now looking to see if CAR T-cells designed to attach to the CD70 protein on cancer cells can help treat advanced RCC.
Adjuvant therapy
Doctors are studying whether giving additional (adjuvant) treatment after surgery can help lower the risk of kidney cancer coming back, especially when there’s a higher risk that this might happen. For example, getting the immunotherapy drug pembrolizumab (Keytruda) for about a year has been shown to help lower this risk in some people, especially those with clear cell RCC. Other medicines are being studied as well to see if they can lower this risk.
Neoadjuvant therapy
Doctors are also studying giving drug treatments, such as immunotherapy or targeted therapy , before surgery (called neoadjuvant therapy).
- For some people whose cancer can be removed, it might shrink the tumor(s). This might let the doctor do a less extensive surgery, and it might help lower the risk that the cancer will come back.
- For some people whose tumors can’t be removed completely with surgery, it might shrink the tumor enough that surgery can be done.
Studies are being done to see which people might benefit the most from neoadjuvant therapy (as well as which drugs might be most effective in this setting).
Tests and other factors to help determine treatment options
Along with looking for new medicines and the best ways to combine and use existing ones, a major area of research is finding better ways to choose the best treatment for each person.
Researchers are looking at whether certain lab test results or other factors might make a person's cancer more (or less) likely to respond to certain medicines. Knowing this can help doctors give treatments that are more likely to be helpful, while avoiding giving those that aren’t (but that could still have side effects).
Risk Factors for Kidney Cancer
A risk factor is anything that increases your chance of getting a disease such as cancer. Different cancers have different risk factors. Some risk factors, like smoking, can be changed. Others, like your age or family history, can’t be changed.
Having a risk factor, or even several risk factors, does not mean that you will get the disease. And some people who get the disease may have few or no known risk factors.
Several risk factors could make you more likely to develop kidney cancer. But, even if a person with kidney cancer has a risk factor, it is often very hard to know how much that risk factor contributed to the cancer.
Smoking
Smoking increases the risk of renal cell carcinoma (RCC), the most common type of kidney cancer. The increased risk seems to be related to how much you smoke. The risk drops slowly over time if you stop smoking.
Excess body weight
People who have excess body weight have a higher risk for RCC. Having excess body weight may cause changes in certain hormones that can lead to RCC.
High blood pressure
The risk of kidney cancer is higher in people with high blood pressure. This risk does not seem to be lowered even if someone is taking medicines to treat the high blood pressure.
Family history of kidney cancer
People with a strong family history of RCC (even without one of the known inherited conditions listed below) have a higher chance of developing cancer. This risk is highest for people who have a brother or sister with kidney cancer. This might be due to shared genes, something that both family members were exposed to in a shared environment, or both.
Workplace exposure to certain chemicals
Many studies have suggested that being exposed to certain substances at work, such as trichloroethylene or cadmium, increases the risk for RCC.
Being male
RCC is about twice as common in men as in women. This might be because men are more likely to smoke and historically have been more likely to be exposed to cancer-causing chemicals at work.
Race and ethnicity
In the US, kidney cancer rates are highest among American Indian and Alaska Native people. They are slightly higher among African American people than among White people. The reasons for this are not clear.
Certain pain medicines
Some studies have suggested that long-term use of pain medicines such as acetaminophen (and possibly aspirin) may be linked to an increased risk of RCC.
Advanced kidney disease
People with advanced kidney disease, especially those needing dialysis, have a higher risk of RCC. (Dialysis is a treatment used to remove toxins from your body if your kidneys aren’t working properly.)
Genetic and hereditary risk factors
Some people inherit gene changes from their parents that can increase their risk of RCC. Sometimes this might be the only effect of a gene change. But sometimes it might lead to an inherited syndrome that increases a person’s risk of other health issues (including other cancers), as well.
People who have hereditary conditions linked to RCC must see their doctors often, especially if they have already been diagnosed with RCC. Some doctors might recommend regular imaging tests (such as CT scans) to look for new kidney tumors in these people.
Most of the conditions listed here result in a much higher risk for getting kidney cancer, although they account for only a small portion of kidney cancers overall.
Von Hippel-Lindau disease
People with von Hippel-Lindau disease often develop several kinds of tumors and cysts (fluid-filled sacs) in different parts of the body. They have an increased risk for developing clear cell RCC, especially at a younger age. They may also have benign tumors in their eyes, brain, spinal cord, pancreas, and other organs; and a type of adrenal gland tumor called pheochromocytoma.
This condition is caused by mutations (changes) in the VHL gene.
Hereditary papillary renal carcinoma
People with hereditary papillary renal carcinoma tend to develop one or more papillary RCCs, but they do not have tumors in other parts of the body, as is the case with the other inherited conditions listed here. This disorder is usually linked to changes in the MET gene.
Hereditary leiomyomatosis and renal cell cancer
People with this syndrome often develop smooth muscle tumors called leiomyomas (fibroids) of the skin and uterus (in women) and have a higher risk for developing papillary RCCs.
This condition has been linked to changes in the FH gene.
Birt-Hogg-Dubé (BHD) syndrome
People with this syndrome tend to develop many small benign skin tumors and have an increased risk of different kinds of kidney tumors, including RCCs and oncocytomas. They may also have benign or malignant tumors of several other tissues.
This condition has been linked to changes in the FLCN gene.
Hereditary paraganglioma-pheochromocytoma syndromes
People with these syndromes often develop neuroendocrine tumors called paragangliomas , including tumors in the adrenal gland called pheochromocytomas. They also have an increased risk of RCC.
These conditions are caused by defects in genes such as SDHA, SDHB, and SDHC, and SDHD.
Cowden syndrome
People with Cowden syndrome have a high risk of breast, thyroid, and kidney cancers.
This condition is linked to changes in the PTEN gene.
Tuberous sclerosis
People with tuberous sclerosis develop many, usually benign (non-cancerous) tumors in different parts of the body including the skin, brain, lungs, eyes, kidneys, and heart. Although the kidney tumors are most often benign, occasionally they can be clear cell RCC.
This condition is linked to defects in the genes TSC1 and TSC2.
BAP1 tumor predisposition syndrome
People with this condition have an increased risk for some types of skin cancers, as well as melanomas of the eye, mesotheliomas, clear cell RCC, and possibly other cancers. This condition is caused by changes in the BAP1 gene.
Sickle cell trait and disease
Some people inherit a change in a gene that codes for hemoglobin, the protein in red blood cells that helps them carry oxygen. People who inherit this gene change from one parent have sickle cell trait (SCT), but usually don’t have obvious symptoms from it. People who inherit gene changes from both parents have sickle cell disease (SCD).
People with either SCT or SCD have an increased risk of renal medullary carcinoma (RMC). This rare subtype of RCC most often occurs in younger people, tends to grow quickly, and can be hard to treat.
The increased risk of RMC is thought to be caused by changes in the SMARCB1 gene.
What Causes Kidney Cancer?
Cancer is caused by changes in the DNA inside our cells. DNA carries our genetic information (genes), which controls how our cells function. Our DNA, which comes from both our parents, affects more than just how we look.
Changes (mutations) in genes
Some genes normally help control when our cells grow, divide to make new cells, or repair mistakes in DNA, or they cause cells to die when they’re supposed to. If these genes aren’t working properly, it can lead to cells growing out of control. For example:
- Changes in genes that normally help cells grow, divide, or stay alive can lead to these genes being more active than they should be, causing them to become oncogenes. These genes can result in cells growing out of control.
- Genes that normally help keep cell division under control or cause cells to die at the right time are known as tumor suppressor genes. Changes that turn off these genes can result in cells growing out of control.
- Some genes normally help repair mistakes in a cell’s DNA. Changes that turn off these DNA repair genes can result in the buildup of DNA changes within a cell, which might lead to them growing out of control.
Any of these types of DNA changes might lead to cells growing out of control and forming a tumor.
Changes in several genes are usually needed to cause kidney cancer. These changes can either be inherited from a parent or they can be acquired during a person’s lifetime.
Inherited gene mutations
Certain inherited gene changes can run in some families and increase the risk of kidney cancer. The inherited syndromes these changes cause lead to a small portion of all kidney cancers.
For example, the VHL gene is a tumor suppressor gene. It normally helps keep cells from growing out of control. Mutations (changes) in this gene can be inherited from parents, leading to von Hippel-Lindau (VHL) disease. When the VHL gene is mutated, it is no longer able to control the abnormal growth, and kidney cancer is more likely to develop.
Inherited changes in the following tumor suppressor genes also lead to an increased risk of kidney cancer:
- The FH genes (linked to hereditary leiomyomas which can cause fibroids in the skin and uterus)
- The FLCN gene (as seen in Birt-Hogg-Dube syndrome)
- The SDHA, SDHB, SDHC, and SDHD genes (as seen in the paraganglioma-pheochromocytoma syndromes)
People with hereditary papillary renal carcinoma have inherited changes in the MET oncogene that cause it to be turned on all the time. This can lead to uncontrolled cell growth and make a person more likely to develop papillary RCC.
Special genetic tests can detect some of the gene mutations linked with these inherited syndromes. If you have a family history of kidney cancer or other cancers linked to these syndromes, you may want to ask your doctor about genetic counseling and genetic testing.
Genetic testing can be complex. Before having it done, it’s important to speak with a qualified cancer genetics professional. They can explain how testing might help you, how it is done, its limitations, and what the results might mean.
Acquired gene mutations
Some gene mutations happen during a person’s lifetime and are not passed on. They affect only cells that come from the original mutated cell. These changes are called acquired mutations. In most people with kidney cancer, the gene mutations that lead to cancer are acquired rather than having been inherited.
Certain risk factors, such as exposure to cancer-causing chemicals, might play a role in causing some of these acquired mutations. For example, when people smoke, the lungs absorb many of the cancer-causing chemicals in tobacco smoke into the bloodstream. Because the kidneys filter this blood, many of these chemicals become concentrated in the kidneys. Several of these chemicals are known to damage kidney cells in ways that can cause the cells to become cancer.
Excess body weight, another risk factor for kidney cancer, alters the balance of some of the body’s hormones. Researchers are now learning how certain hormones help control the growth (both normal and abnormal) of many different tissues in the body, including the kidneys.
However, many of the acquired gene changes that can lead to kidney cancer can be just random events that sometimes happen inside a cell, without having an outside cause.
Most people with non-inherited clear cell RCC have changes in the VHL tumor suppressor gene in their cancer cells that have caused the gene to stop working properly. These gene changes are acquired during a person's life.
Other gene changes may also cause renal cell carcinomas. Researchers continue to look for these changes.
Can Kidney Cancer Be Prevented?
There is no sure way to prevent kidney cancer. Some kidney cancer risk factors , such as your race/ethnicity and family history, can’t be controlled. But there are some things you can do that might lower your risk of kidney cancer.
Cigarette smoking is responsible for a large percentage of kidney cancers, so if you smoke, stopping may help lower your risk.
Excess body weight is also a risk factor for renal cell cancer. Doing your best to stay at a healthy weight by being active and eating a healthy diet may help lower your chances of getting kidney cancer.
Avoiding exposure to harmful substances such as trichloroethylene at work may also reduce your risk for renal cell cancer.
Can Kidney Cancer Be Found Early?
Many kidney cancers are found while they are still just in the kidney, but others are found at a more advanced stage. There are a few reasons for this:
- Sometimes these cancers can grow quite large without causing any pain or other problems.
- Because the kidneys are deep inside the body, small kidney tumors cannot be seen or felt during a physical exam.
- There are no recommended screening tests for kidney cancer for people at average risk.
For people at average risk of kidney cancer
Some tests can find some kidney cancers early, but none of these is recommended to screen for kidney cancer in people at average risk.
A routine urine test (urinalysis), which is sometimes part of a medical checkup, might find small amounts of blood in the urine of some people with early kidney cancer. But blood in the urine can also have other causes, including urinary tract or bladder infections, bladder cancer, and benign (non-cancerous) kidney conditions such as kidney stones. Sometimes people with kidney cancer don’t have blood in their urine until the cancer is quite large or has already spread to other parts of the body.
Imaging tests such as computed tomography (CT) scans and magnetic resonance imaging (MRI) can often find small kidney cancers. But these tests can be time-consuming and expensive. Ultrasound is less expensive and can also detect some early kidney cancers. But it’s not clear that the benefits of screening for kidney cancer with any of these tests would outweigh the possible downsides. Another issue with these types of tests is that they can’t always tell benign tumors from small kidney cancers. This could mean that a person might need to get other types of tests, such as a biopsy, even if it turns out they don’t have kidney cancer.
Often, kidney cancers are found by accident when imaging tests are done for some other reason. These cancers usually do not cause pain or other symptoms when they are found. The survival rate for these kidney cancers is very high because they are usually found at a very early stage.
For people at increased risk of kidney cancer
People who have certain inherited conditions have a higher risk of kidney cancer. This includes syndromes such as:
- von Hippel-Lindau disease
- Hereditary papillary renal carcinoma
- Hereditary leiomyomatosis and renal cell carcinoma
- Birt-Hogg-Dubé syndrome
- Paraganglioma-pheochromocytoma syndromes
- BAP1 tumor predisposition syndrome
- Tuberous sclerosis
Doctors often recommend that people with these types of conditions get regular physical exams, possibly along with CT, MRI, or ultrasound scans, usually starting when they are young, to look for kidney tumors (and possibly other types of tumors). Kidney cancers that are found early this way can often be cured.
Some doctors also recommend that people with kidney diseases treated by long-term dialysis, who are also at increased risk, have regular tests to look for kidney cancer.
Genetic counseling and testing for people who might be at increased risk of kidney cancer
It’s important to tell your doctor if any of your family members (blood relatives) have had kidney cancer, especially at a younger age, or if they have been diagnosed with an inherited condition linked to this cancer, such as von Hippel-Lindau disease. Your doctor may recommend that you consider genetic counseling and testing to see if you have one of these conditions.
Before having genetic tests, it’s important to talk with a genetic counselor so that you understand what the tests can − and can’t − tell you, how the tests are done, and what any results would mean. Genetic tests are used to look for the gene mutations that cause these inherited conditions, not kidney cancer itself. Your risk may be increased if you have one of these conditions, but it does not mean that you have (or definitely will get) kidney cancer.
Kidney Cancer Signs and Symptoms
Early kidney cancers often don’t cause any signs or symptoms, but larger or more advanced ones might. Some possible signs and symptoms of kidney cancer include:
- Blood in the urine (hematuria)
- Low back pain on one side (not caused by injury)
- A mass (lump) on the side or lower back
- Fever that is not caused by an infection and that doesn’t go away
- Fatigue (feeling very tired)
- Loss of appetite
- Weight loss
- Anemia (low red blood cell counts)
These signs and symptoms can be caused by kidney cancer (or another type of cancer), but more often they are caused by other, benign (non-cancerous) diseases. For example, blood in the urine is most often caused by a bladder or urinary tract infection or a kidney stone. Still, if you have any of these symptoms, see a doctor so that the cause can be found and treated, if needed.
Tests for Kidney Cancer
Kidney cancer (also known as renal cell cancer, or RCC) might be found because of signs or symptoms a person is having, or it might be found because of lab tests or imaging tests a person is getting for some other reason.
The actual diagnosis of kidney cancer is made by looking at a sample of kidney cells in the lab or sometimes by how the kidney looks on an imaging test.
If you think you have possible signs or symptoms of kidney cancer, it’s important to see a doctor.
Medical history and physical exam
If you have any signs or symptoms that suggest you might have kidney cancer, your doctor will want to take your complete medical history to check for possible risk factors and to learn more about your symptoms.
A physical exam can provide information about possible signs of kidney cancer and other health problems you might have. For example, the doctor may be able to feel an abnormal mass (lump) when they examine your abdomen (belly).
If symptoms or the results of the physical exam suggest you might have kidney cancer, you will probably need to have certain tests. These might include lab tests, imaging tests, or biopsies of the kidney.
Blood tests
Lab tests can’t show for sure if a person has kidney cancer, but they can sometimes give the first hint that there may be a kidney problem.
If cancer has already been diagnosed, blood tests are also done to get a sense of a person’s overall health and to help determine if the cancer might have spread to other areas. They can also help show if a person is healthy enough to have surgery.
Complete blood count (CBC): This test measures the levels of different cells in the blood, which are often abnormal in people with kidney cancer. Anemia (having too few red blood cells) is very common. Less often, a person may have too many red blood cells (called polycythemia). People with kidney cancer sometimes have high levels of blood platelets or of neutrophils, which are a type of white blood cell. Blood counts are also important to make sure a person is healthy enough for surgery.
Blood chemistry tests: These tests are often done in people who might have kidney cancer, because the cancer can affect the levels of certain chemicals in the blood. For example, high levels of calcium, lactate dehydrogenase (LDH), or liver enzymes are sometimes found. Levels of other chemicals, such as alkaline phosphatase, are also routinely tested. Blood chemistry tests also measure kidney function, which is especially important if certain imaging tests or surgery are planned.
Urine tests
Your urine may be tested if your doctor suspects a kidney problem.
Urinalysis: This test looks at the levels of different chemicals and proteins in a urine sample. The samples can also be checked for red or white blood cells and other substances that can’t be seen with the naked eye. Many people with kidney cancer will have blood in their urine, sometimes in very small amounts. Other parts of this test can help show how well the kidneys and some other organs are working.
Urine cytology: If an imaging test (see below) shows that a person has a tumor in the renal pelvis (the middle part of the kidney, which is attached to the ureter), a test called urine cytology might be done. This test looks for cancer cells in the urine.
Imaging tests to look for kidney cancer
Imaging tests use x-rays, magnetic fields, sound waves, or radioactive substances to create pictures of the inside of your body. Imaging tests might be done for some reasons, such as:
- To look for tumors in people who have symptoms or abnormal test results that might be from kidney cancer
- To help guide a needle biopsy (see below)
- To learn how far a kidney cancer has spread
- To help determine if treatment is working
- To look for possible signs of cancer coming back (recurring) after treatment
Unlike most other types of cancer, doctors can sometimes diagnose kidney cancer with a fair amount of certainty using only imaging tests, without needing a biopsy (removing a sample of the tumor). But this isn’t always the case, and often people need a biopsy to make sure their diagnosis is correct.
Computed tomography (CT) scan
A CT scan uses x-rays to make detailed cross-sectional images of your body. A CT scan of the abdomen (belly) can provide precise information about the size, shape, and location of a kidney tumor. CT scans can also show if a cancer has spread to nearby lymph nodes or to other parts of the body, such as the lungs.
A special type of CT scan, known as CT angiography, might sometimes be done to get a better look at the blood vessels around the kidney.
CT-guided needle biopsy: If a kidney biopsy is needed, this test can also be used to guide a biopsy needle into the mass (lump) to get a sample to check for cancer.
When a CT is done to look at the kidneys, an IV (intravenous) contrast dye is often needed to make certain areas stand out better on the scan. This could cause problems in people whose kidneys aren’t working well in the first place, so another type of imaging test, such as an ultrasound or MRI, might be done instead. Your kidney function will be checked with a blood test before you get a CT scan with IV contrast.
Magnetic resonance imaging (MRI)
MRIs use radio waves and strong magnets to create detailed images of the body. MRIs aren’t usually the first imaging test done to look for kidney cancer, but they may be done:
- If a person can’t get the contrast dye for a CT scan because they have an allergy to it or they don’t have good kidney function.
- If there’s a chance that the cancer has grown into major blood vessels in the abdomen, like the inferior vena cava. (MRIs provide a better picture of blood vessels than CT scans.)
- To look at abnormal areas in the brain and spinal cord that might be due to cancer spread.
A special type of MRI, known as MR angiography, might sometimes be done to get a better look at the blood vessels around the kidney.
Ultrasound
Ultrasound uses sound waves and their echoes to create pictures of organs in the body. It can help find a kidney mass and show if it is solid or filled with fluid. (Solid masses are more likely to be tumors.) Different ultrasound patterns can also help doctors tell the difference between some types of benign and malignant (cancerous) kidney tumors.
Ultrasound-guided needle biopsy: If a kidney biopsy is needed, ultrasound can also be used to guide a biopsy needle into the mass to take a sample.
Chest x-ray
An X-ray of the chest may be done if kidney cancer has been diagnosed to see if the cancer has spread to the lungs. More often, though, a CT scan of the chest is done instead because it can show more detail.
Bone scan
A bone scan can help show if cancer has spread to your bones. For this test, you will get an injection of a small amount of low-level radioactive material. It will collect mainly in abnormal areas of bone. A special camera is then used to find the levels of radioactivity in different parts of your body.
This test is only likely to be done if there is reason to think the cancer might have spread to the bones, such as if a person is having bone pain or if blood tests show an increased calcium or alkaline phosphatase level.
Kidney biopsy
For most other types of cancer, a biopsy is needed to confirm the diagnosis. During a biopsy, small pieces of a suspicious area are removed and checked in the lab for cancer cells.
But if kidney cancer is suspected, imaging tests can often provide enough information for a surgeon to decide if surgery is needed. The diagnosis is then confirmed when part or all of the kidney is removed and is looked at in the lab.
There are some situations in which a kidney biopsy might be needed:
- When the results of imaging tests aren’t clear enough to know if surgery is needed.
- To confirm a tumor is cancer if a person might not be treated with surgery, such as with small tumors that will be watched and not treated, or when other treatments are being considered.
When a biopsy is needed, it’s typically done by inserting a thin, hollow needle into the tumor and removing a sample of cells (known as a needle biopsy). Often a CT scan or ultrasound is used to help guide the needle into the tumor.
If the doctor thinks the kidney cancer might have spread to other parts of the body, they may biopsy one of these areas instead of the kidney.
Lab tests on biopsy and surgery samples
Samples from any biopsies (or from surgery to remove all or part of the kidney) are sent to a lab, where they are looked at by a pathologist, a doctor specially trained in diagnosing diseases with lab tests.
If kidney cancer cells are found, further tests might be done to determine which type of kidney cancer it is.
Another important feature is the grade of the cancer, specifically called the Fuhrman grade. This is based on how much the cancer cells look like those of normal kidney cells. Renal cell cancers are usually graded on a scale of 1 to 4.
- Grade 1 cancers have cells that look a lot like normal kidney cells. These cancers usually grow and spread slowly and tend to have a good prognosis (outlook).
- Grade 4 renal cell cancers look very different from normal kidney cells. These cancers tend to have a worse prognosis.
The kidney cancer's grade is one of the factors in deciding on a treatment.
Kidney Cancer Stages
After someone is diagnosed with kidney cancer, doctors will try to figure out whether it has spread, and if so, how far. This process is called staging. The stage describes how much cancer is in the body. It helps determine how serious the cancer is and how best to treat it. Doctors also use a cancer's stage when talking about survival statistics.
How is the kidney cancer stage determined?
The stages of kidney cancer range from I (1) through IV (4).
- The lower the number, the less the cancer has spread.
- A higher number, such as stage IV, means cancer has spread more.
Although other factors can also be important, cancers with similar stages tend to have a similar outlook and are often treated in much the same way.
The stage of a kidney cancer can be determined in 2 ways:
The clinical stage is based on the results of physical exams, imaging tests, and any biopsies that have been done (see Tests for Kidney Cancer). The clinical stage can be helpful in determining treatment options.
If surgery is done, the pathological stage (also called the surgical stage) can be determined. This is based on the information above, as well as what is learned about the cancer by examining tissue removed during the operation.
The clinical and pathological stages for kidney cancer are the same, but it’s possible that the stage might change after surgery is done. For example, surgery might show that the cancer has spread farther than what was seen on imaging tests. If this is the case, the pathological stage might be higher than the clinical stage.
The staging system most often used for kidney cancer is the American Joint Committee on Cancer (AJCC) TNM system. The TNM system is based on 3 key pieces of information:
- The size and extent of the main (primary) tumor (T): How large is the tumor? Has it has grown into nearby areas?
- The spread to nearby lymph nodes (N): Has the cancer spread to nearby lymph nodes?
- The spread (metastasis) to distant sites (M): Has the cancer spread to other organs such as the bones, brain, or lungs?
Numbers or letters after T, N, and M provide more details about each of these factors. Higher numbers mean the cancer is more advanced. Once a person’s T, N, and M categories have been determined, this information is combined in a process called stage grouping to assign an overall stage.
Kidney cancer staging can be complex. If you have any questions about your stage, please ask your doctor to explain it to you in a way you understand.
- Adrenal glands: Small glands that sit on top of each kidney. Like the kidneys, they are covered by Gerota’s fascia.
- Gerota’s fascia: A thin, fibrous layer of tissue that surrounds each kidney and adrenal gland.
- Renal vein: The main vein leaving the kidney, which connects it to the inferior vena cava.
- Inferior vena cava: The large vein that carries blood from the lower parts of the body back up to the heart.
|
Stage |
Stage grouping |
Stage description* |
|
I |
T1 N0 M0 |
The main tumor is no more than 7 centimeters ( 7 cm; a little less than 3 inches) across and is only in the kidney (T1). The cancer has not spread to nearby lymph nodes (N0) or distant organs (M0). |
|
II |
T2 N0 M0 |
The main tumor is larger than 7 cm across but is still only in the kidney (T2). The cancer has not spread to lymph nodes (N0) or distant organs (M0). |
|
III
|
T3 N0 or N1 M0 |
The main tumor is growing into a major vein (like the renal vein or the inferior vena cava) or into tissue around the kidney, but it is not growing into the adrenal gland or beyond Gerota’s fascia (T3). The cancer might or might not have spread to nearby lymph nodes (N0 or N1) but it hasn't spread to distant organs (M0). |
|
OR |
||
|
T1 to T2 N1 M0 |
The main tumor can be any size, but it hasn't grown outside the kidney (T1 or T2). The cancer has spread to nearby lymph nodes (N1) but has not spread to distant lymph nodes or other organs (M0). |
|
|
IV |
T4 Any N M0 |
The main tumor is growing beyond Gerota’s fascia and may be growing into the adrenal gland on top of the kidney (T4). The cancer might or might not have spread to nearby lymph nodes (any N). It has not spread to distant lymph nodes or other organs (M0). |
|
OR |
||
|
Any T Any N M1 |
The main tumor can be any size and may have grown outside the kidney (any T). It may or may not have spread to nearby lymph nodes (any N). It has spread to distant lymph nodes and/or other organs (M1). |
|
*The following additional categories are not listed in the table above:
- T0: There is no evidence of a primary tumor.
- NX: Nearby lymph nodes cannot be assessed due to lack of information.
Prognostic systems for advanced kidney cancer
For stage IV (metastatic) renal cell carcinoma, factors other than the stage of the cancer can also be important.
Doctors have developed systems that use some of these factors to put people into risk groups, which can help determine a person’s prognosis (outlook) and treatment options.
The two systems that are commonly used are the Memorial Sloan Kettering Cancer Center (MSKCC) model and the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) criteria.
These two systems use 5 or 6 factors to put people into low-, intermediate-, and high-risk groups.
The factors in the MSKCC system include:
- High blood lactate dehydrogenase (LDH) level
- High blood calcium level
- Anemia (low red blood cell count)
- Less than a year from diagnosis to the need for systemic treatment (targeted therapy, immunotherapy, or chemotherapy)
- Poor performance status (a measure of how well a person can do normal daily activities)
The factors in the IMDC system include:
- High neutrophil count
- High blood platelet cell count
- High blood calcium level
- Anemia (low red blood cell count)
- Less than a year from being diagnosed to needing systemic treatment (targeted therapy, immunotherapy, or chemotherapy)
- Poor performance status (a measure of how well a person can do normal daily activities)
For each system, people with:
- None of the above factors are considered to be in the low-risk
- 1 or 2 factors are considered to be in the intermediate-risk group
- 3 or more of these factors are considered to be in the high-risk group
A person’s risk group status can be used to help decide which treatment options might be best.
Questions to Ask About Kidney Cancer
It’s important to have honest, open discussions with your cancer care team. They want to answer all your questions, so that you can make informed treatment and life decisions. For instance, consider these questions:
When you’re told you have kidney cancer
- What type of kidney cancer do I have?
- Where is the cancer located?
- Has the cancer spread beyond where it started?
- What is the stage of the cancer, and what does that mean for me?
- Will I need other tests before we can decide on treatment?
- Do I need to see any other doctors or health professionals?
- Is there a chance I have an inherited condition that increased my risk of kidney cancer? Should I consider genetic testing?
- If I’m concerned about the costs and insurance coverage for my diagnosis and treatment, who can help me?
When deciding on a treatment plan
- What are my treatment options?
- What do you recommend and why?
- Does my cancer need to be treated right away, or can it be watched closely ?
- How much experience do you have treating this type of cancer?
- Should I get a second opinion? How do I do that? Can you recommend a doctor or cancer center?
- What would the goal of treatment be?
- How quickly do we need to decide on treatment? What should I do to be ready for treatment?
- How long will treatment last? What will it be like? Where will it be done?
- What risks or side effects are there to the treatments you suggest? Are there things I can do to reduce the side effects?
- How might treatment affect my daily activities? Can I still work full time?
- What are the chances the cancer will recur (come back) with these treatment plans?
- What will we do if the treatment doesn’t work or if the cancer recurs?
- What if I have trouble getting to and from my treatments because of transportation problems?
During treatment
Once treatment begins, you’ll need to know what to expect and what to look for. Not all of these questions may apply to you, but asking the ones that do may be helpful.
- How will we know if the treatment is working?
- Is there anything I can do to help manage side effects?
- What symptoms or side effects should I tell you about right away?
- How can I reach someone on the team on nights, holidays, or weekends?
- Do I need to change what I eat during treatment?
- Are there any limits on what I can do?
- Can I exercise during treatment? If so, what kind of exercise should I do, and how often?
- If I start to feel overwhelmed, depressed, or distressed, can you suggest a mental health professional I can see?
- What if I need some social support during treatment?
After treatment
- Do I need to follow a special diet after treatment?
- Are there any limits on what I can do?
- What symptoms should I watch for?
- What type of follow-up will I need after treatment?
- How often will I need to have follow-up exams and imaging tests?
- How will we know if the cancer has come back? What should I watch for?
Along with these sample questions, be sure to write down some of your own. For instance, you might want more information about recovery times so you can plan your work or activity schedule. You might also want to ask about clinical trials for which you may qualify.
Keep in mind that doctors are not the only ones who can provide you with information. Other health care professionals, such as nurses and social workers, may have the answers to some of your questions. You can find more information about communicating with your health care team in The Doctor-Patient Relationship.
Local treatments
Local therapies treat the tumor but don’t affect the rest of the body. They are more likely to be useful for earlier stage (less advanced) cancers, although they might also be used in some other situations.
Surgery for Kidney Cancer
Surgery is often part of the main treatment for kidney cancer. Sometimes it might be the only treatment that’s needed, especially for cancers that are still only in the kidney.
Types of surgery for kidney cancer
Depending on the stage and location of the cancer and other factors, different types of surgery might be done.
- For tumors in the kidney, surgery might be done to remove the entire kidney (known as a radical nephrectomy) or the just the part of the kidney with the tumor (known as a partial nephrectomy).
- Sometimes, nearby lymph nodes might also be removed (known as a lymphadenectomy).
- If the cancer has spread (metastasized), sometimes surgery might be an option to remove the tumor(s) in another part of the body.
Some people whose cancer has spread to other organs may be helped by surgery to take out the kidney tumor. This might also help with symptoms such as pain or bleeding.
Radical nephrectomy
In this operation, the surgeon removes the kidney, the fatty tissue and Gerota’s fascia around the kidney, and some nearby lymph nodes. Sometimes the adrenal gland on top of the kidney is removed as well, especially if there’s a high risk of the cancer spreading there (such as if there’s a larger tumor in the upper part of the kidney).
Open radical nephrectomy
This operation is done through a single, long incision in the skin to reach the kidney.
The surgeon can make the incision in several places. The most common places are the middle of the abdomen (belly), under the ribs on the same side as the cancer, or in the back, just behind the kidney. Each approach has its benefits in treating cancers of different sizes and in different parts of the kidney.
If the tumor has grown from the kidney through the renal vein (the vein leading away from the kidney) and into the inferior vena cava (the large vein that carries blood from the lower part of the body back up to the heart), the heart may need to be stopped for a short time in order to remove the tumor. The patient is put on cardiopulmonary bypass (a heart-lung machine) that circulates their blood while bypassing their heart. If you need this, a heart surgeon will work with your urologist during your operation.
Laparoscopic nephrectomy and robotic-assisted laparoscopic nephrectomy
These operations are done through several small incisions instead of one large one. If a radical nephrectomy is needed, many doctors and patients now prefer to use these methods when they can be done.
Laparoscopic nephrectomy: For this approach, the surgeon inserts special long, thin instruments through the small incisions to remove the kidney. One of the instruments is a laparoscope, which is a long tube with a small video camera on the end. This lets the surgeon see inside the abdomen. Usually, one of the incisions has to be made longer toward the end of the operation to remove the kidney (although it’s not as long as the incision for an open radical nephrectomy).
Robotic-assisted laparoscopic nephrectomy: In this approach, the surgeon sits at a panel near the operating table and controls robotic arms with long, thin surgical instruments on the ends. The robotic system lets the surgeon move the instruments more easily and with more precision than during standard laparoscopic surgery.
Both types of laparoscopic surgery are complex and take time for surgeons to learn. If you are considering either type of laparoscopic surgery, be sure to find a surgeon with a lot of experience.
In experienced hands, either type of laparoscopic nephrectomy is about as effective as an open radical nephrectomy. The main benefits of these approaches are that they usually result in a shorter hospital stay, a faster recovery time, and less pain after surgery. However, the laparoscopic approach may not be a good option for larger tumors or for tumors that have grown into the renal vein or spread to lymph nodes around the kidney.
Partial nephrectomy (nephron-sparing surgery)
In a partial nephrectomy, the surgeon removes only the part of the kidney that contains the cancer, leaving the rest of the kidney in place. The benefit of this approach is that the person keeps more kidney function. Studies have shown the long-term results from partial nephrectomy are about the same as when the whole kidney is removed.
For people with early-stage kidney cancer, a partial nephrectomy might be a good option if:
- The kidney tumor is smaller – usually less than about 10 centimeters (about 4 inches) across, and it isn’t in the central part of the kidney.
- A person already has (or is likely to have) reduced kidney function, for example if they only have one working kidney, if they have tumors in both kidneys, if they’re at risk for some type of chronic kidney disease, or if they have an inherited condition that increases their risk of more kidney tumors later on.
A partial nephrectomy might not be an option if:
- The tumor is very large.
- The tumor is in the central part of the kidney.
- There is more than one tumor in the same kidney.
- The tumor has reached the renal vein or inferior vena cava, or the cancer has spread to the lymph nodes or distant organs.
Partial nephrectomy typically is a more complex operation than a radical nephrectomy, so it should only be done by a doctor with experience.
As with a radical nephrectomy, this operation can be done in different ways.
Open partial nephrectomy
For an open partial nephrectomy, the surgeon operates through one long incision in the skin. The surgeon can make the incision in several places, depending on factors like the location of the tumor.
Laparoscopic partial nephrectomy and robotic-assisted laparoscopic partial nephrectomy
These operations are done through several small incisions instead of one large one.
Laparoscopic partial nephrectomy: For this approach, the surgeon inserts special long, thin instruments through the small incisions to remove the kidney. One of the instruments is a laparoscope, which is a long tube with a small video camera on the end that lets the surgeon see inside the abdomen.
Robotic-assisted laparoscopic partial nephrectomy: In this approach, the surgeon sits at a panel near the operating table and controls robotic arms with long, thin surgical instruments on the ends. The surgeon can move the instruments more easily and with more precision than during standard laparoscopic surgery.
Done by an experienced surgeon, either type of laparoscopic partial nephrectomy is about as effective as an open partial nephrectomy. The main benefits of these approaches are that they usually result in a shorter hospital stay, a faster recovery time, and less pain after surgery.
However, both types of laparoscopic partial nephrectomy are complicated operations, and the laparoscopic approach may not be a good option for more complex kidney tumors.
It also takes time for surgeons to learn how to do these operations. If you are considering either type of laparoscopic surgery, be sure to find a surgeon with experience.
Lymphadenectomy (lymph node removal)
In this procedure, the surgeon removes nearby lymph nodes to see if they contain cancer. Some lymph nodes near the kidney are often removed as part of a radical nephrectomy.
A more extensive lymphadenectomy in which more lymph nodes are removed (known as a lymph node dissection) may be done if the tumor has features suggesting it is at high risk of spreading to the nodes, such as if it has a higher grade. Lymph nodes are also removed if they look enlarged on imaging tests or feel abnormal during the operation.
Some doctors might also remove these lymph nodes to check them for cancer spread, even when they aren’t enlarged, to help better stage the cancer. This might affect whether a person should get further (adjuvant) treatment after surgery.
Removal of metastases
In some people with kidney cancer, the cancer has already spread (metastasized) to other parts of the body by the time it’s found. The most common sites of spread are the lungs, lymph nodes, bones, and liver. For some people, surgery to remove these tumors may still be helpful.
Attempting a surgical cure
If the cancer has spread to very few spots outside the kidney that can all be removed safely, surgery to remove these tumors may lead to long-term survival in some people.
The metastasis may be removed at the same time as a radical nephrectomy or later if the cancer recurs (comes back).
Surgery to relieve symptoms (palliative surgery)
If other treatments are no longer helpful, surgery might be done to help relieve pain or other symptoms caused by tumors, although this type of surgery isn’t intended to cure the cancer.
Risks and side effects of surgery
The short-term risks of any type of surgery include reactions to anesthesia, bleeding (which might require blood transfusions), blood clots, and infections. Most people will have at least some pain after the operation, which can usually be helped with pain medicines, if needed.
Other possible risks of surgery include:
- Damage to organs and blood vessels (such as the spleen, pancreas, aorta, vena cava, or large or small bowel) during surgery
- Pneumothorax (unwanted air in the chest space around the lungs)
- Incisional hernia (bulging of internal organs near the surgical incision due to problems with wound healing)
- Leakage of urine into the abdomen (after partial nephrectomy)
- Kidney failure (if the remaining kidney fails to function well)
Ask your doctor what to expect after surgery. You might want to ask about your recovery time, if there are any limits on what you can do, common side effects to watch out for, and when you should contact someone on your cancer care team if you’re having problems.
Ablation and Other Local Therapy for Kidney Cancer
Whenever possible, surgery is the main treatment for kidney cancer that can be removed. But for some people, such as those who aren’t healthy enough for surgery, other treatments can sometimes be used to destroy (ablate) the kidney tumor.
These methods are usually only considered for small kidney tumors (typically no larger than 4 cm or about 1½ inches across).
These treatments might be helpful for some people, although there is much less data on how well they work over time than there is for surgery.
Cryotherapy (cryoablation)
Cryotherapy uses extreme cold to destroy a tumor.
For this treatment, a hollow probe (needle) is inserted into the tumor, either through the skin (percutaneously) or during laparoscopy (in which a long, thin tube with a tiny video camera on the end is inserted into the abdomen through a small incision). Very cold gases are then passed through the probe, creating an ice ball at its tip that destroys the tumor.
To be sure the tumor is destroyed without too much damage to nearby tissues, the doctor carefully watches images of the tumor during the procedure (with ultrasound, CT, or MRI scans) or measures the temperature of the nearby tissues.
The type of anesthesia used for cryotherapy depends on how the procedure is being done.
Possible side effects include bleeding and damage to the kidneys or other nearby organs.
Radiofrequency ablation (RFA)
Radiofrequency ablation (RFA) uses high-energy radio waves to heat and destroy the tumor. A thin, needle-like probe is placed through the skin and moved forward until the end is in the tumor. Placement of the probe is guided by ultrasound or CT scan. Once it is in place, an electric current is passed through the tip of the probe. This heats the tumor and destroys the cancer cells.
RFA is usually done as an outpatient procedure, using local anesthesia (numbing medicine) where the probe is inserted. You may be given medicine to help you relax as well.
Major complications are uncommon, but they can include bleeding and damage to the kidneys or other nearby organs.
Other local treatments
Other, newer types of local treatments might also be used to destroy tumors in the kidney (or possibly in other parts of the body). These approaches haven’t been around as long as cryotherapy or RFA, so there’s less long-term data on them at this point. Still, they might be options for some people.
Microwave ablation
For this treatment, imaging tests are used to guide a needle-like probe (antenna) into the tumor. Electromagnetic microwaves are then created at the tip of the probe to heat to destroy the tumor.
Stereotactic ablative body radiotherapy (SABR)
Also known as stereotactic body radiation therapy (SBRT), this is a type of advanced radiation therapy. Imaging tests are used to guide thin beams of radiation at the tumor from many different angles. SABR can usually be given throughout a few treatments.
Irreversible electroporation
For this treatment, imaging tests are used to guide long needles (electrodes) into place around the tumor. The needles are then used to create a strong electrical field within the tumor. This causes holes (pores) to form in the walls of the cancer cells, leading to their death.
Active Surveillance for Kidney Cancer
Not all kidney tumors need to be treated right away. Some small kidney tumors turn out to be benign (not cancer). And even many small kidney cancers tend to grow slowly, without spreading.
One option for some people with small kidney tumors may be to watch the tumor carefully to see if it grows, without treating it right away. This is usually done with regular imaging tests (ultrasound, CT or MRI scans) of the abdomen (belly). Blood tests and imaging tests of the chest might be done at times as well. If the tumor starts growing quickly or shows other worrisome signs, it can then be removed with surgery or treated another way.
Sometimes, the tumor might be biopsied to help determine if it is cancer or not. This could help determine if surveillance is a reasonable option or if the tumor needs to be treated.
Active surveillance might be a good choice for people who are older or who have other serious health problems, as it can allow them to avoid the risks of treatments such as surgery or ablation.
If a biopsy hasn’t been done, watching the tumor closely for a while can also give the doctor a better idea of whether it is likely to be cancer, based on how fast it is growing.
This approach doesn’t use heat or cold to destroy the cells, and it might prove to be useful in areas where it’s important to protect vital structures like nearby blood vessels. But many doctors feel that more research is needed to show it is safe and effective.
Radiation Therapy for Kidney Cancer
Radiation therapy uses high-energy rays or particles to kill cancer cells.
When is radiation therapy used for kidney cancer?
Radiation therapy isn’t usually the first treatment for kidney cancer. But it might be an option if:
- The cancer is still only in the kidney, but a person isn’t healthy enough for (or doesn’t want to have) surgery or has only one kidney. Sometimes other ablative treatments might be tried before radiation.
- The cancer has spread, but there are no more than a few tumors in other parts of the body. Radiation might be an option to try to destroy these tumors, although other treatments, such as surgery or other ablative techniques, might be options as well.
- The cancer returns after treatment, especially if it has spread more widely. In this situation, radiation might be an option to help relieve (palliate) symptoms caused by tumors in some parts of the body, such as the brain or bones. This type of treatment is known as palliative radiation therapy.
How is radiation therapy given?
When radiation therapy is used to treat kidney cancer, a special machine is used to create and focus beams of radiation at the tumor. This type of treatment is known as external beam radiation therapy (EBRT).
Each treatment is much like getting an X-ray, although the radiation dose is stronger. The treatment itself is painless and typically lasts only a few minutes, although the setup time, getting you into place for treatment, takes longer.
When treating a tumor in the kidney or a small area of cancer spread (such as a single tumor in a lung), radiation is usually given as stereotactic body radiation therapy (SBRT), also known as stereotactic ablative body radiotherapy (SABR).
For this advanced type of EBRT, imaging tests are used to guide the delivery of thin beams of radiation to a precise area, such as a kidney tumor, from many different angles. Large doses of radiation can be given in each dose, so the entire course of treatment can often be given in just a few days.
SBRT is often known by the names of the machines that deliver the radiation, such as Gamma Knife, X-Knife, CyberKnife, or Clinac.
Possible side effects of radiation therapy
Side effects of radiation therapy might include:
- Skin changes (similar to sunburn) and hair loss where the radiation passes through the skin
- Nausea or diarrhea (when radiation is aimed at the abdomen)
- Feeling tired
Other side effects are also possible, depending on where the radiation is aimed.
Most side effects go away shortly after treatment is finished, but some might last longer.
Radiation may also make side effects from some other treatments worse.
If you’re getting radiation, ask a member of your cancer care team what side effects to expect.
Targeted Drug Therapy for Kidney Cancer
As researchers have learned more about the gene and protein changes inside cells that cause them to become cancer cells, they have developed drugs that target some of these changes. These targeted drugs are different from standard chemotherapy (chemo) drugs. They tend to work better against kidney cancer than standard chemo drugs, and they often have different side effects.
When are targeted drugs used for kidney cancer?
Treating advanced kidney cancer
Targeted drugs are used mainly to treat advanced kidney cancer. One of these drugs is typically part of the first treatment for advanced cancers, often along with an immunotherapy drug.
Many different targeted drugs can be used to treat kidney cancer. If one doesn’t work, another can be tried. It’s not yet clear if one sequence of drugs is better than another. Studies are being done to help answer this.
Adjuvant therapy after surgery
The targeted drug sunitinib (Sutent) might also be an option as an adjuvant treatment after surgery to remove the kidney, to help lower the risk that the cancer will come back.
Targeted drugs used to treat kidney cancer
Most of the targeted drugs used to treat kidney cancer work by blocking proteins called tyrosine kinases inside cancer cells that normally help them grow, or that help them create new blood vessels that feed the tumor. Drugs that target these types of proteins are known as tyrosine kinase inhibitors, or TKIs.
Drugs that target tumor blood vessel growth (angiogenesis)
Sunitinib (Sutent)
Sunitinib acts by blocking both angiogenesis and several tyrosine kinases in cancer cells that are important for their growth and survival.
This drug is a pill taken daily, typically for 4 weeks on and 2 weeks off. Some doctors might recommend taking it 2 weeks on and 1 week off to reduce side effects.
Sunitinib can be used in people with advanced kidney cancer. It might also be an option after surgery in people with a high risk of their cancer returning, to help lower the risk that the cancer will come back, although an immunotherapy drug such as pembrolizumab (Keytruda) is more likely to be used in this situation instead.
The most common side effects of sunitinib are:
- Nausea
- Diarrhea
- Changes in skin or hair color
- Mouth sores
- Weakness
- Low white and red blood cell counts
Other possible effects include feeling tired, high blood pressure, heart problems, bleeding, hand-foot syndrome, and low thyroid hormone levels.
Pazopanib (Votrient)
Pazopanib blocks several tyrosine kinases involved in cancer cell growth, as well as the formation of new blood vessels in the tumor. This drug is a pill, typically taken once a day.
Common side effects of pazopanib include:
- High blood pressure
- Nausea
- Diarrhea
- Headaches
- Low blood cell counts
- Hair color change
It can cause abnormal liver function test results, but it rarely leads to severe liver damage that could be life-threatening. Problems with bleeding, clotting, and wound healing can occur, as well.
In rare cases, it can also cause a problem with the heart rhythm or even heart failure. If you are taking this drug, your doctor will monitor your heart with EKGs as well as check your blood tests for liver or other problems.
Cabozantinib (Cabometyx)
Cabozantinib blocks several tyrosine kinases that help cancer cells grow and survive, as well as some that help form new blood vessels in the tumor.
This drug can be used to treat advanced kidney cancer, either by itself or along with the immunotherapy drug nivolumab (Opdivo). It is taken as a pill, typically once a day.
Common side effects of cabozantinib include:
- Diarrhea
- Fatigue (feeling tired)
- Nausea and vomiting
- Poor appetite and weight loss
- High blood pressure
- Hand-foot syndrome
- Constipation
Less common but more serious side effects can include serious bleeding, blood clots, very high blood pressure, severe diarrhea, and holes forming in the intestines.
Lenvatinib (Lenvima)
Lenvatinib is a tyrosine kinase inhibitor that helps block new blood vessels from forming in the tumor, as well as targeting some of the proteins in cancer cells that normally help them grow.
This drug can be used along with the immunotherapy drug pembrolizumab in people with advanced kidney cancer. It can also be used with the targeted drug everolimus (see below). Lenvatinib is a capsule typically taken once a day.
Common side effects of lenvatinib include:
- Diarrhea
- Fatigue
- Joint or muscle pain
- Loss of appetite
- Nausea and vomiting
- Mouth sores
- Weight loss
- High blood pressure
- Swelling in the arms or legs
Less common but more serious side effects can include serious bleeding, blood clots, very high blood pressure, severe diarrhea, holes forming in the intestines, and kidney, liver, or heart failure.
Bevacizumab (Avastin)
Bevacizumab works by slowing the growth of new blood vessels. It can be used to treat advanced kidney cancer, either alone or along with another drug. It is most often used after other drug treatments have been tried.
It is given by infusion into a vein (IV), typically once every 2 weeks.
More common side effects of bevacizumab include:
- High blood pressure
- Feeling tired
- Headaches
Less common but possibly serious side effects include bleeding, blood clots, holes forming in the intestines, heart problems, and slow wound healing.
Axitinib (Inlyta)
Axitinib blocks several tyrosine kinases that help form new blood vessels in the tumor.
This drug can be used alone or with certain immunotherapy drugs, like pembrolizumab or avelumab, as a treatment for people with advanced kidney cancer. Axitinib is a pill, typically taken twice a day.
Common side effects of axitinib include:
- High blood pressure
- Fatigue (feeling tired)
- Nausea and vomiting
- Diarrhea
- Poor appetite
- Weight loss
- Voice changes
- Hand-foot syndrome
- Constipation
- Changes in liver and thyroid function (which can be seen on lab tests)
A small number of people develop blood pressure high enough to be life-threatening. This drug can also cause problems with bleeding, clotting, and wound healing.
Tivozanib (Fotivda)
Tivozanib blocks several tyrosine kinases involved in cancer cell growth and the formation of new blood vessels in the tumor.
This drug can be used in people with advanced kidney cancer.
Tivozanib is a pill, typically taken daily for 3 weeks followed by 1 week off. This cycle is then repeated for as long as the drug is still helpful.
Common side effects of tivozanib include:
- High blood pressure
- Diarrhea
- Nausea
- Poor appetite
- Cough
- Mouth sores
- Feeling tired
- Voice changes
Less common but more serious side effects can include heart problems, life threatening high blood pressure, blood clots, bleeding, poor wound healing, abnormal thyroid tests, and damage to the kidney.
Belzutifan (Welireg)
Belzutifan is a HIF inhibitor. It blocks a protein called hypoxia-inducible factor 2 alpha (HIF-2a), which is involved in both cancer cell growth and new blood vessel formation in tumors.
Belzutifan can be used:
In people with von Hippel-Lindau (VHL) disease who have kidney cancer and don’t need surgery right away.
- In people with advanced kidney cancer that has already been treated with a different targeted drug and with an immune checkpoint inhibitor (a type of immunotherapy drug).
This drug is taken as pills, typically once a day.
Common side effects of belzutifan include:
- Low red blood cell counts (anemia)
- Feeling tired and/or dizzy
- Nausea
- Headache
- Increased blood sugar levels
- Changes in lab tests show the drug might be affecting the kidneys
Less common but more serious side effects can include very low red blood cell counts (severe anemia, which might require blood transfusions), and low oxygen levels in the body, for which you might need oxygen therapy or even be admitted to the hospital.
Drugs that target the mTOR protein
Temsirolimus (Torisel)
Temsirolimus works by blocking a protein known as mTOR, which normally helps cells grow and divide.
This drug can be used to treat advanced kidney cancers. It is usually used after other drug treatments have been tried. Temsirolimus is given by intravenous (IV) infusion, typically once a week.
The most common side effects of temsirolimus include:
- Skin rash
- Weakness
- Mouth sores
- Nausea
- Loss of appetite
- Fluid buildup in the face or legs
- Increases in blood sugar and cholesterol levels
Rarely, it can cause more serious side effects.
Everolimus (Afinitor)
Everolimus also blocks the mTOR protein.
This drug can be used to treat advanced kidney cancers. It can be used by itself or along with the targeted drug lenvatinib (see above), typically after at least one other drug treatment has been tried.
Everolimus is taken as a pill, typically once a day.
Common side effects of everolimus include
- Mouth sores
- An increased risk of infections
- Nausea
- Loss of appetite
- Diarrhea
- Skin rash
- Feeling tired or weak
- Fluid buildup (usually in the legs)
- Increases in blood sugar and cholesterol levels
A less common but serious side effect is lung damage, which can cause shortness of breath or other problems.
Immunotherapy for Kidney Cancer
Immunotherapy is the use of medicines to boost a person's own immune system to recognize and destroy cancer cells more effectively. Different types of immunotherapy can be used to treat kidney cancer.
Immune checkpoint inhibitors
An important part of the immune system is its ability to keep itself from attacking normal cells in the body. To do this, it uses “checkpoint” proteins on immune cells, which act like switches that need to be turned on (or off) to start an immune response. Kidney cancer cells sometimes use these checkpoints to avoid being attacked by the immune system. But these drugs target the checkpoint proteins, which help the immune system attack the cancer cells.
PD-1 and PD-L1 inhibitors
Pembrolizumab (Keytruda) and Nivolumab (Opdivo) are drugs that target PD-1, a protein on immune system cells (called T cells) that normally help keep these cells from attacking other cells in the body. Avelumab (Bavencio) targets PD-L1, a protein that binds to PD-1, which is found on some tumor cells and immune cells. By blocking either of these checkpoint proteins, these drugs boost the immune response against kidney cancer cells. This can often shrink tumors or slow their growth.
These drugs can be used in different situations to treat kidney cancer:
- Pembrolizumab can be given as an adjuvant treatment after surgery in people who are at higher risk of their cancer coming back, to help lower this risk. It is usually given for about a year after surgery.
- One of these drugs is often part of the first treatment for advanced kidney cancer, along with a targeted drug or with the CTLA-4 inhibitor ipilimumab (see below).
- One of these drugs can also be used if advanced kidney cancer starts growing again after other drug treatments have been tried. It might be given alone or along with another type of drug.
These drugs are given by infusion into a vein (IV), typically once every 2 to 6 weeks, depending on the drug.
Possible side effects of PD-1 and PD-L1 inhibitors
The most common side effects of these drugs include:
- Fatigue (feeling tired)
- Cough
- Nausea
- Itching
- Skin rash
- Loss of appetite
- Constipation
- Joint pain
- Diarrhea
See below for possible severe side effects of all checkpoint inhibitors. While it’s not common, these drugs can also have more serious side effects - see below.
CTLA-4 inhibitor
Ipilimumab (Yervoy) is another drug that boosts the immune response, but it blocks CTLA-4, a different checkpoint protein on T cells that normally helps keep them in check.
Ipilimumab is not used by itself, but with nivolumab (a PD-1 inhibitor – see above).
Ipilimumab is given by intravenous (IV) infusion, usually once every 3 weeks for 4 treatments.
Possible side effects of CTLA-4 inhibitors
Side effects tend to be more common with this drug than with the PD-1 and PD-L1 inhibitors discussed above. The most common side effects from ipilimumab include:
- Fatigue
- Diarrhea
- Skin rash
- Itching
This drug can also have more serious side effects - see below.
Possible serious side effects of all checkpoint inhibitors
Serious side effects aren’t common with these drugs, but they are possible.
Infusion reactions: Some people might have an infusion reaction while getting one of these drugs. This is like an allergic reaction, and can include fever, chills, flushing of the face, rash, itchy skin, feeling dizzy, wheezing, and trouble breathing. It’s important to tell your doctor or nurse right away if you have any of these symptoms while getting this drug.
Autoimmune reactions: These drugs work by removing one of the safeguards on the body’s immune system. Sometimes the immune system starts attacking other parts of the body, which can cause serious or even life-threatening problems in the lungs, intestines, liver, hormone-making glands (like the thyroid), kidneys, or other organs.
It’s very important to report any new side effects during or after treatment to your health care team right away. If serious side effects do occur, you may need to stop treatment and take high doses of corticosteroids to suppress your immune system.
Cytokines
Cytokines are small proteins in the body that boost the immune system. Man-made versions of cytokines, such as interleukin-2 (IL-2), might sometimes be used to treat kidney cancer in very specific cases. They can shrink kidney cancers in a small percentage of people.
Interleukin-2 (IL-2)
In the past, IL-2 was often used as a first-line treatment for advanced kidney cancer, and it may still be helpful for some people. But the newer immune checkpoint inhibitors (see above) and targeted drugs are more likely to be helpful.
IL-2 is given by infusion through a vein (IV). Giving high doses of IL-2 seems to offer the best chance of shrinking the cancer, but this can cause serious side effects, so it is not used in people who are in poor overall health.
Side effects of IL-2 can include flu-like symptoms, such as fever, chills, aches, severe tiredness, drowsiness, and low blood cell counts. In high doses, IL-2 can cause fluid to build up in the body so that the person swells up and can feel very sick.
Because these side effects can be severe, high-dose IL-2 is only given in the hospital at certain centers that are experienced with giving this type of treatment.
Chemotherapy for Kidney Cancer
Chemotherapy (chemo) uses anti-cancer drugs that are given into a vein (IV) or taken by mouth (as pills). These drugs enter your bloodstream and reach nearly all areas of the body, which makes this treatment potentially useful for cancer that has spread (metastasized) to organs beyond the kidney.
When is chemotherapy used for kidney cancer?
The most common types of kidney cancer (renal cell carcinoma, or RCC), such as clear cell RCC, typically don’t respond well to chemo, so it’s not usually part of the treatment for these cancers. Targeted drugs and immunotherapy are the most common treatments for most advanced kidney cancers.
However, chemo can be helpful for some less common types of RCC, including collecting duct RCC and renal medullary carcinoma. Usually, a platinum drug (cisplatin or carboplatin) is combined with either gemcitabine or paclitaxel to treat these cancers. These drugs are given by infusion into a vein (IV).
Doctors give chemotherapy in cycles, with each period of treatment followed by a rest period to allow the body time to recover. Chemo cycles generally last a few weeks.
Possible side effects of chemotherapy
Chemo drugs can also affect other cells in the body, which can lead to certain side effects.
The side effects of chemo depend on which drugs are given, the doses used, and the length of treatment. Possible side effects of chemo can include:
- Hair loss
- Mouth sores
- Loss of appetite
- Nausea and vomiting
- Diarrhea or constipation
- Increased chance of infections (due to low white blood cell counts)
- Easy bruising or bleeding (due to low blood platelet counts)
- Fatigue (feeling tired due to low red blood cell counts)
These side effects usually go away after treatment is finished. There are often ways to prevent or lessen them. For example, medicine can be given to help prevent or reduce nausea and vomiting.
Some chemo drugs can also cause other side effects. For example, drugs like cisplatin, carboplatin, and paclitaxel can damage nerves. This can sometimes lead to symptoms (mainly in the hands and feet) such as pain, burning or tingling, sensitivity to cold or heat, or weakness. This is called peripheral neuropathy.
Ask your health care team about the side effects your chemo drugs may cause.
Treatment of Kidney Cancer by Stage
The type of treatment(s) your doctors recommend will depend mainly on the stage of the kidney cancer and on your overall health and decisions. Other factors, such as type and grade of the cancer, might also affect your treatment options.
This section sums up the options usually considered for each stage of renal cell carcinoma (RCC), the most common type of kidney cancer.
Treating stage I or II kidney cancer
Stage I and II cancers are still only in the kidney.
Active surveillance
Some small (stage I) cancers might not need to be treated right away. Small tumors often grow slowly, and some might never cause serious problems. Because of this, active surveillance might be an option for some people with small kidney tumors. With this approach, the tumor is watched closely with regular imaging tests (such as CT scans or ultrasounds) and possibly other tests, and it’s only treated if it grows or starts to show other concerning signs.
Surgery
If treatment is needed, these cancers are usually removed with surgery when possible.
- Partial nephrectomy (removing the part of the kidney containing the cancer) is often the treatment of choice for smaller tumors. This is especially true for people have reduced kidney function (or who might have it in the future).
- Radical nephrectomy (removing the entire kidney) is often favored if the tumor is larger, if it’s in the central part of the kidney, or if there’s more than one tumor in the kidney.
Some lymph nodes near the kidney are often removed as well. More lymph nodes might need to be removed if any of them look enlarged on imaging tests, or if there’s a higher risk that the cancer might spread to the nodes. Most often, no further treatment is needed after surgery.
If, after surgery, the cancer cells are found to have troubling features when evaluated in the lab (such as being very high grade), one option might be to get adjuvant (additional) treatment to help lower the risk of the cancer coming back. Most often, this is with the immunotherapy drug pembrolizumab (Keytruda), which is given for about a year.
Other treatment options
For people who aren’t healthy enough to have surgery or who don't want surgery, other local treatments such as cryotherapy or radiofrequency ablation (RFA) can sometimes be used to destroy (ablate) the kidney tumor. Radiation therapy (particularly stereotactic body radiation therapy, or SBRT) may be another option. Although these types of treatments can have outcomes similar to surgery as far as the chances of the cancer spreading to other parts of the body, some studies show the cancer might be more likely to come back in the same area.
Treating stage III kidney cancer
Stage III cancers have grown into nearby large veins or tissues around the kidney, and/or they have spread to nearby lymph nodes.
Surgery
Surgery is typically the main treatment for these cancers. Most often, this is a radical nephrectomy, in which the entire kidney is removed. A partial nephrectomy (removing the part of the kidney containing the tumor) might also be an option if it’s possible, especially in people with reduced kidney function or who have tumors in both kidneys.
Some lymph nodes near the kidney are often removed as well. More lymph nodes might need to be removed if any of them look enlarged on imaging tests, or if there’s a higher risk that the cancer might spread to the nodes.
If the cancer has grown into the inferior vena cava (the large vein that brings blood from the lower part of the body back up to the heart), your surgeon may need to cut open this vein to remove all of the cancer. This may require putting you on bypass (a heart-lung machine), so that the heart can be stopped for a short time to remove the cancer from the vein.
For clear cell RCC, an option after surgery is to get adjuvant (additional) treatment to help lower the risk of the cancer coming back. Most often, this is with the immunotherapy drug pembrolizumab (Keytruda), which is given for about a year.
Other treatment options
For people who can’t have surgery for some reason, radiation therapy or another type of local treatment might be an option.
Some stage III cancers can’t be removed completely by surgery or destroyed with other treatments. These cancers might get the same treatment as stage IV cancers, with targeted therapy drugs, immunotherapy, or a combination of these.
Treating stage IV kidney cancer
In stage IV kidney cancer, the main tumor has grown outside the kidney, or the cancer has spread to other parts of the body, such as distant lymph nodes or other organs.
Treatment of stage IV kidney cancer depends mainly on how extensive the cancer is and on a person’s general health.
For most people with stage IV kidney cancer, medicines such as immunotherapy and targeted drugs are the main treatments (see below). But in some cases, surgery may still be a part of treatment.
If both the kidney tumor and metastases appear to be removable
While it’s not common, sometimes the main tumor appears to be removable and there is only limited spread to another area (such as to one or a few spots in the lungs). In these situations, surgery to remove both the kidney and the metastasis (the outside area of cancer spread) may be an option if a person is in good enough health. Other options to treat the metastatic tumors might include ablative treatments or radiation therapy.
If all of the tumors are removed (or destroyed), additional (adjuvant) treatment with the immunotherapy drug pembrolizumab might be considered. It is typically given for about a year.
If just the kidney tumor appears to be removable
If the kidney tumor can be removed but the cancer has spread extensively elsewhere, treatment options might include:
- Removing the kidney with the tumor first. This type of surgery (known as a cytoreductive nephrectomy) isn’t recommended for most people, but it might be an option for otherwise healthy people in a low-risk group. Surgery is then followed by drug treatments (immunotherapy and or targeted drugs) for most people.
- Giving drug treatments (immunotherapy and/or targeted drugs) first. This is likely to be preferred for most people, even if it looks like the cancer in the kidney can be removed. For some people, if the cancer shrinks a lot with this treatment, surgery, ablative treatments, or radiation therapy might be options to try to remove or destroy any remaining tumors.
If the kidney tumor isn’t removable
If the kidney tumor can’t be removed, the first treatment is usually with medicines such as immunotherapy and/or targeted therapy drugs. Often, one of each type of drug is part of the first treatment. Which ones are used depends to some extent on if the cancer is a clear cell RCC or a non-clear cell RCC. If one treatment doesn’t work (or stops working), another one can often be tried. For some less common types of non-clear cell RCC, such as collecting duct RCC or renal medullary carcinoma, chemotherapy is often the first treatment.
While it’s not common, sometimes the first treatment might shrink the tumors enough so that surgery, ablative treatments, or radiation might be options to try to get rid of any remaining tumors.
In other situations, surgery or other treatments might be used to help relieve symptoms from the cancer, such as pain or bleeding, rather than trying to get rid of the cancer completely. This type of treatment is called palliative therapy. (You can read more about palliative treatment for cancer in Palliative (Supportive) Care or in Advanced Cancer, Metastatic Cancer, and Bone Metastasis.) If you have advanced kidney cancer and your doctor suggests surgery, ablation, or radiation, be sure you understand what the goal of the treatment is.
No matter what type of treatment you’re getting, having your pain controlled can help you maintain your quality of life. Treating the cancer itself can often help with this. Medicines to relieve pain can also be helpful, and they will not interfere with your other treatments. Controlling any pain you have can often help you be more active and continue your daily activities.
Because advanced kidney cancer is very hard to cure, clinical trials of new combinations of targeted therapy drugs, immunotherapy, or other new treatments are also options.
Treating recurrent kidney cancer
Cancer is called recurrent when it comes back after treatment. Recurrence can be local (near the area of the original tumor), or it may be in distant parts of the body.
Treatment of kidney cancer that comes back (recurs) after initial treatment depends on where it recurs and what treatments have been used, as well as a person’s health and wishes for further treatment.
Local recurrence
For cancers that recur near the area of the original kidney tumor after surgery, further surgery or other localized treatments or radiation might be options. Even if not all of the cancer can be removed or destroyed, these treatments might still help relieve symptoms in some people. Other treatment options will most likely include immunotherapy and/or targeted therapy drugs. Clinical trials of new treatments are an option as well.
Distant recurrence
Kidney cancer that recurs in distant parts of the body is treated like stage IV cancer (see above). Your options will depend on where the cancer is; if it’s thought to be removable or not; which, if any, drugs you received as part of your first treatment (and how long ago you got them); and on your overall health and preferences.
For cancers that continue to grow or spread during treatment with immunotherapy or targeted therapy drugs, different drugs still might be helpful. Recurrent cancers can sometimes be hard to treat, so you might also want to ask your doctor about clinical trials.
For some people with recurrent kidney cancer, palliative treatments may be the best option. These treatments are intended to help control the cancer and relieve any symptoms it is causing. Options might include radiation therapy, ablative treatments, or even some type of surgery, if a person is healthy enough. Controlling symptoms such as pain is also an important part of treatment at any stage of the disease.
Living as a Kidney Cancer Survivor
For some people with kidney cancer, treatment can remove or destroy the cancer. Completing treatment can be both stressful and exciting. You may be relieved to finish treatment, yet it can be hard not to worry about cancer coming back. This is very common if you’ve had cancer.
Sometimes though, the kidney cancer might not go away completely. Some people may get regular treatment with immunotherapy or targeted therapy drugs or other treatments to try to keep the cancer in check. Learning to live with cancer that doesn’t go away can be difficult and very stressful.
Follow-up care
It’s very important to go to all of your follow-up appointments. Even if you have completed treatment, your doctors will still want to watch you closely. During these visits, your doctors will ask if you are having any problems and may order exams and lab tests or imaging tests to look for signs of cancer or treatment side effects.
Almost any cancer treatment can have side effects. Some might only last for a few days or weeks, but others might last a long time. Some side effects might not even show up until years after you have finished treatment. Your doctor visits are a good time to ask questions and talk about any changes or problems you notice or concerns you have.
It’s very important to let your doctor know about any new symptoms or problems you have, so the cause can be found and treated, if needed.
Doctor visits
To some extent, how often you have follow-up visits and tests (and which tests you have) will depend on the stage of your cancer, the treatment you received, and the risk that the cancer might come back.
For people who had early-stage cancer, many doctors recommend follow-up visits (which may include imaging tests and blood tests) a few months after treatment is done, and then about every 12 months for at least a few years after treatment.
For people who were treated for later-stage cancers, follow-up visits with imaging and lab tests most likely will be every 3-6 months for a few years and then once a year.
Some doctors may advise different follow-up schedules.
Ask your doctor for a survivorship care plan
Talk with your doctor about developing a survivorship care plan for you. This plan might include:
- A suggested schedule for follow-up exams and tests
- A schedule for other tests you might need to look for long-term health effects from your cancer or its treatment
- A list of possible late- or long-term side effects from your treatment, including what to watch for and when you should contact your doctor
- Suggestions for things you can do that might improve your health, including possibly lowering your chances of the cancer coming back
- Reminders to keep your appointments with your primary care provider (PCP), who will monitor your general health
Keeping health insurance and copies of your medical records
Even after treatment, it’s very important to keep health insurance. Tests and doctor visits cost a lot, and even though no one wants to think of their cancer coming back, this could happen.
At some point after your cancer treatment, you might find yourself seeing a new doctor who doesn’t know about your medical history. It’s important to keep copies of your medical records to give your new doctor the details of your diagnosis and treatment.
Can I lower my risk of kidney cancer progressing or coming back?
If you have (or have had) kidney cancer, you probably want to know if there are things you can do that might lower your risk of the cancer growing or coming back, such as exercising, eating a certain type of diet, or taking nutritional supplements.
Stopping smoking
If you smoke, it might help to know that some research suggests that stopping smoking is linked to a lower risk of the cancer progressing, as well as to longer survival. Stopping smoking can also help lower your risk of getting another smoking-related cancer.
Other things that might be helpful
At this time, not enough is known about kidney cancer to say for sure if there are other things you can do that will be helpful. Adopting healthy behaviors such as eating well, getting regular physical activity, and staying at a healthy weight might help, but no one knows for sure. Still, we do know that these types of changes can have positive effects on your health that can extend beyond your risk of kidney cancer or other cancers.
About dietary supplements
So far, no dietary supplements (including vitamins, minerals, and herbal products) have been shown to clearly help lower the risk of kidney cancer progressing or coming back. This doesn’t mean that no supplements will help, but it’s important to know that so far, none have been proven to do so.
Dietary supplements are not regulated like medicines in the United States – they don’t have to be proven effective (or even safe) before being sold, although there are limits on what they’re allowed to claim they can do. If you’re thinking about taking any type of nutritional supplement, talk to your healthcare team. They can help you decide which ones you can use safely while avoiding those that might be harmful.
If the cancer comes back
If the cancer does return at some point, your treatment options will depend on where the cancer is, what treatments you’ve had before, and your overall health. Surgery, ablative treatments, radiation therapy, targeted therapy, immunotherapy, or some combination of these might be options. Other types of treatment might also be used to help relieve any symptoms from the cancer.
Getting emotional support
It’s normal to feel depressed, anxious, or worried when kidney cancer is a part of your life. Some people are affected more than others. But everyone can benefit from help and support from other people, whether friends and family, religious groups, support groups, professional counselors, or others.
Second cancers after treatment
People who’ve had cancer can be affected by several health problems, but often a major concern is facing cancer again. Cancer that comes back after treatment is called a recurrence. But some cancer survivors may develop a new, unrelated cancer later. This is called a second cancer.
Unfortunately, being treated for kidney cancer doesn’t mean you can’t get another cancer. People who have had kidney cancer can still get the same types of cancers that other people get. In fact, they might be at a higher risk of certain types of cancer, including:
- A second kidney cancer (This is different from the first cancer coming back.) The risk of a second kidney cancer is highest in people who were diagnosed before age 50.
- Bladder cancer
- Cancer of the ureter (the tube that connects the kidney to the bladder)
- Prostate cancer (in men)
- Thyroid cancer
- Melanoma of the skin
Can I lower my risk of getting a second cancer?
There are steps you can take to lower your risk of getting another cancer and staying as healthy as possible. For example, smoking is linked to an increased risk of kidney cancer and several other cancers, so if you smoke, stopping might help lower your risk for some of these cancers. Kidney cancer survivors should also try to:
- Get to and stay at a healthy weight.
- Keep physically active and limit the time you spend sitting or lying down.
- Follow a healthy eating pattern that includes plenty of fruits, vegetables, and whole grains, and that limits or avoids red and processed meats, sugary drinks, and highly processed foods.
- Avoid or limit alcohol. If you do drink, have no more than 1 drink per day for women or 2 drinks per day for men.
These steps might also lower your risk of many other health problems.